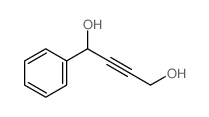
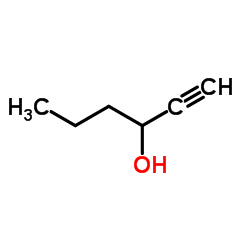
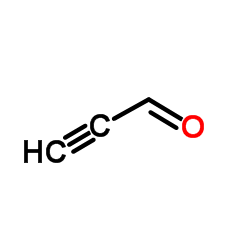
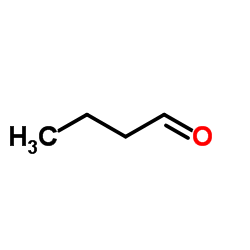
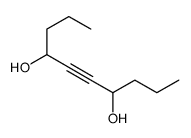
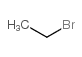
|
~% |
|
~53% |
|
~56% |
|
~39% |
|
~51% |
|
~% |
|
~43% |