| Structure | Name/CAS No. | Articles |
|---|---|---|
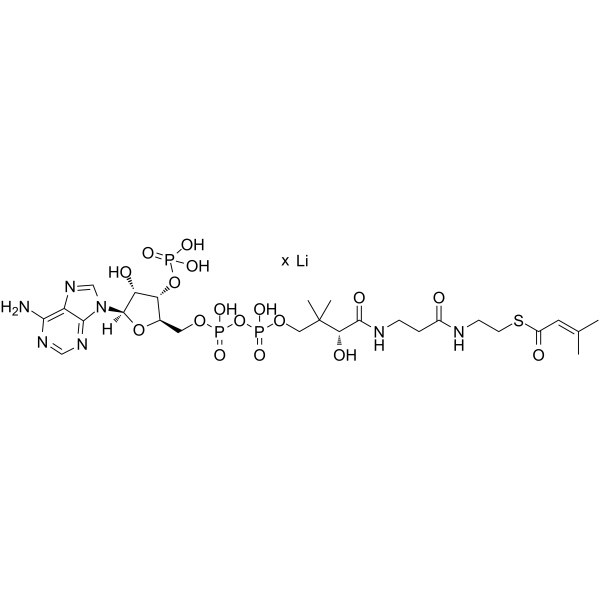 |
β-Methylcrotonyl coenzyme A lithium
CAS:108347-83-7 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
β-Methylcrotonyl coenzyme A lithium
CAS:108347-83-7 |