Retigabine for the adjunctive treatment of adults with partial-onset seizures in epilepsy with and without secondary generalization : a NICE single technology appraisal.
Dawn Craig, Stephen Rice, Fiona Paton, David Fox, Nerys Woolacott
Index: Pharmacoeconomics 31(2) , 101-10, (2013)
Full Text: HTML
Abstract
The National Institute for Health and Clinical Excellence (NICE) invited the manufacturer of retigabine (GlaxoSmithKline) to submit evidence for the clinical and cost effectiveness of this drug for the treatment of adults with partial-onset seizures in epilepsy, with and without secondary generalization, as part of the Institute's single technology appraisal (STA) process. The Centre for Reviews and Dissemination was commissioned to act as the Evidence Review Group (ERG). The ERG undertakes a critical review of the clinical and cost-effectiveness evidence of the technology based upon the manufacturer's submission to NICE. The ERG also independently searches for relevant evidence and evaluates modifications to the manufacturer's decision-analytic model. This paper provides a description of the company submission, the ERG review and NICE's subsequent decisions. The clinical effectiveness data were derived from three placebo-controlled randomized controlled trials (RCTs). A meta-analysis pooling across all doses of retigabine found beneficial effects of retigabine in terms of responder rate (odds ratio [OR] 2.79; 95 % CI 2.08, 3.76) and rate of seizure freedom (OR 2.54; 95 % CI 0.92, 6.98) [both double-blind phase analyses]. When compared in a network meta-analysis with the selected comparator antiepileptic drugs (AEDs) [eslicarbazepine acetate, lacosamide, pregabalin, tiagabine and zonisamide], retigabine offered broadly similar efficacy in terms of responder rate and freedom from seizure. The de novo decision-analytic model presented within the submission evaluated the cost effectiveness of retigabine compared with these AEDs and no treatment (i.e. maintenance therapy). After numerous additional analyses, the ERG considered the use of retigabine to be not cost effective for NICE at thresholds below £43,000 if no treatment was considered a relevant comparator. The NICE Appraisal Committee decided that an appropriate comparator was an active treatment. The Committee recommended that retigabine is offered as an option for the adjunctive treatment of partial-onset seizures with or without secondary generalization in adults aged 18 years and older with epilepsy, only when previous treatment with carbamazepine, clobazam, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, sodium valproate and topiramate has not provided an adequate response, or has not been tolerated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
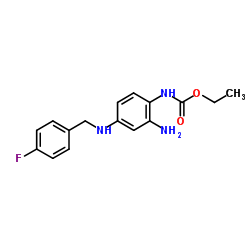 |
Retigabine
CAS:150812-12-7 |
C16H18FN3O2 |
|
Enhanced in vitro CA1 network activity in a sodium channel β...
2014-04-01 [Epilepsia 55(4) , 601-8, (2014)] |
|
Safety profile of two novel antiepileptic agents approved fo...
2013-11-01 [Expert Opin. Drug Saf. 12(6) , 847-55, (2013)] |
|
Ezogabine (retigabine) and its role in the treatment of part...
2012-09-01 [Clin. Ther. 34(9) , 1845-56.e1, (2012)] |
|
[Retigabine - a new antiepileptic drug with a different mech...
2013-01-01 [Postepy. Hig. Med. Dosw. 67 , 973-81, (2013)] |
|
P-retigabine: an N-propargyled retigabine with improved brai...
2015-01-01 [Mol. Pharmacol. 87(1) , 31-8, (2015)] |