Peptide research
1991-01-01
Semiautomated T-bag peptide synthesis using 9-fluorenyl-methoxycarbonyl strategy and benzotriazol-1-yl-tetramethyl-uronium tetrafluoroborate activation.
A G Beck-Sickinger, H Dürr, G Jung
Index: Pept. Res. 4(2) , 88-94, (1991)
Full Text: HTML
Abstract
Multiple peptide synthesis using the T-bag method is considerably facilitated using 9-fluorenyl-methoxycarbonyl strategy and benzotriazol-1-yl-tetramethyl-uronium tetrafluoroborate activation. Convenient protocols were achieved when semiautomation was applied. Successful syntheses of analogues of neuropeptide Y demonstrate the advantages of these improvements. Various peptides consisting of up to eighteen residues were built up with unusual amino acid residues. Useful hints are given for semiautomated multiple peptide synthesis for up to 120 different peptides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
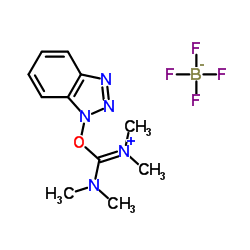 |
TBTU
CAS:125700-67-6 |
C11H16BF4N5O |
Related Articles:
More...
|
Novel polymyxin derivatives carrying only three positive cha...
2008-09-01 [Antimicrob. Agents Chemother. 52 , 3229-36, (2008)] |
|
S. Zimmer et al., C.H. Schneider and A.N. Eberle, eds.
[Proc. 22nd Eur. Pept. Symp.: Peptides 1992 Leiden 1993, p. 393] |
|
O-(BENZOTRIAZOL-1-YL)-N, N, N', N'-TETRAMETHYL...
[Synlett 6 , 709-712, (1999)] |
|
I. Abdelmoty
[Lett. Pept. Sci. 1 , 57, (1994)] |
|
Automated solid-phase peptide synthesis: use of 2-(1H-benzot...
1992-02-01 [Anal. Biochem. 200(2) , 301-9, (1992)] |