| Structure | Name/CAS No. | Articles |
|---|---|---|
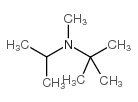 |
n-isopropyl-n-methyl-tert-butylamine
CAS:85523-00-8 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
n-isopropyl-n-methyl-tert-butylamine
CAS:85523-00-8 |