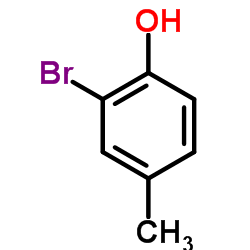
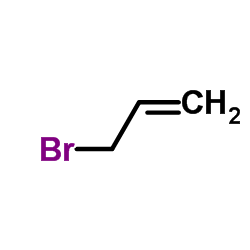
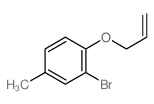
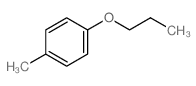
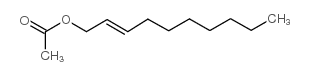
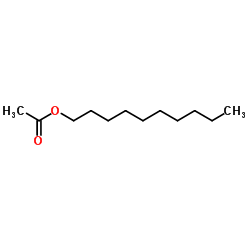
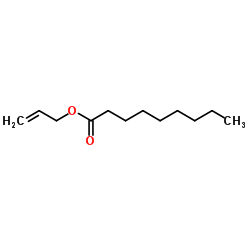
|
~97% |
|
~% |
|
~0% |
|
~97% |
|
~% |
|
~% |
|
~19% |