A sequential high-yielding large-scale solution-method for synthesis of philanthotoxin analogues.
Petrine Wellendorph, Jerzy W Jaroszewski, Steen Honoré Hansen, Henrik Franzyk
Index: Eur. J. Med. Chem. 38(1) , 117-22, (2003)
Full Text: HTML
Abstract
A general, improved procedure for rapid synthesis of philanthotoxin analogues, a pharmacologically important class of polyamine conjugates, is described. The solution-phase procedure is illustrated by gram-scale synthesis of philanthotoxins PhTX-343 and PhTX-12. Selectively protected polyamines are coupled to N(alpha)-Fmoc-protected amino acid pentafluorophenyl esters. After removal of the N(alpha)-Fmoc group, the amine is coupled with carboxylic acid pentafluorophenyl esters. Deprotection followed by a rapid and efficient purification by vacuum liquid chromatography on octadecylsilyl silica (RP-18 phase) gave the philanthotoxin analogues in 74-78% overall yield.Copyright 2002 Editions scienctifiques et médicales Elsevier SAS
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
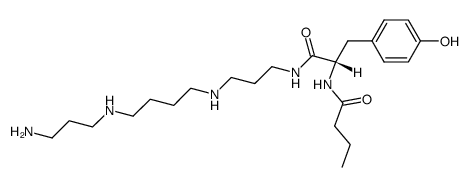 |
Philanthotoxin 343
CAS:115976-93-7 |
C23H41N5O3 |
|
Open-channel blockade is less effective on GluN3B than GluN3...
2012-07-05 [Eur. J. Pharmacol. 686(1-3) , 22-31, (2012)] |
|
Neuroactive polyamine wasp toxins: nuclear magnetic resonanc...
1996-01-19 [J. Med. Chem. 39(2) , 515-21, (1996)] |
|
Potent and voltage-dependent block by philanthotoxin-343 of ...
1997-09-01 [Br. J. Pharmacol. 122(2) , 379-85, (1997)] |
|
Possible influence of intramolecular hydrogen bonds on the t...
2000-01-01 [Receptors Channels 7(3) , 227-36, (2000)] |
|
NMDA receptor subtype selectivity: eliprodil, polyamine spid...
1995-05-01 [J. Neurochem. 64(5) , 2043-8, (1995)] |