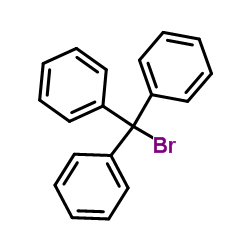
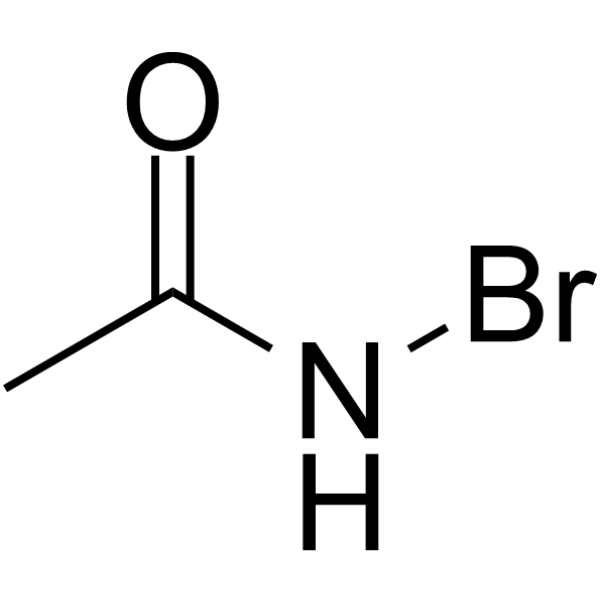
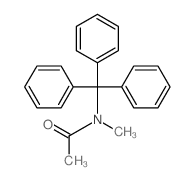
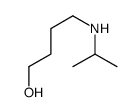
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
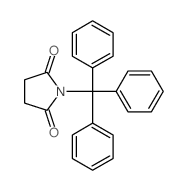
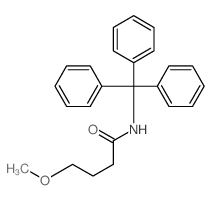
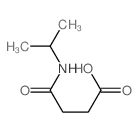
![Butanoic acid,4-oxo-4-[(triphenylmethyl)amino] Structure](https://image.chemsrc.com/caspic/429/6622-12-4.png)