| Structure | Name/CAS No. | Articles |
|---|---|---|
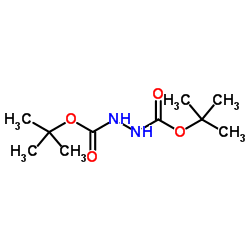 |
Di-Tert-Butyl Hydrazo dicarboxylate
CAS:16466-61-8 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Di-Tert-Butyl Hydrazo dicarboxylate
CAS:16466-61-8 |