Caged phospho-amino acid building blocks for solid-phase peptide synthesis.
Deborah M Rothman, M Eugenio Vazquez, Elizabeth M Vogel, Barbara Imperiali
Index: J. Org. Chem. 68(17) , 6795-8, (2003)
Full Text: HTML
Abstract
Three 1-(2-nitrophenyl)ethyl-caged phospho-amino acids have been synthesized for use in standard N(alpha)-fluorenylmethoxycarbonyl-based solid-phase peptide synthesis (SPPS). The most common naturally occurring phospho-amino acids, serine, threonine, and tyrosine, were prepared as protected caged building blocks by modification with a unique phosphitylating reagent. In previous work, caged phospho-peptides were made using an interassembly approach (Rothman, D. M.; Vazquez, M. E.; Vogel, E. M.; Imperiali, B. Org. Lett. 2002, 4, 2865-2868). However, this technique is limited to creating peptides without oxidation sensitive residues C-terminal to the amino acid to be modified and the methodology involves synthetic manipulations on the solid phase that may limit the utilization of the methodology. Herein we report the facile synthesis of N-alpha-Fmoc-phospho(1-nitrophenylethyl-2-cyanoethyl)-L-serine 1, N-alpha-Fmoc-phospho(1-nitrophenylethyl-2-cyanoethyl)-L-threonine 2, and N-alpha-Fmoc-phospho(1-nitrophenylethyl-2-cyanoethyl)-L-tyrosine 3. These building blocks allow the synthesis of any caged phospho-peptide sequence using standard Fmoc-based SPPS procedures.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
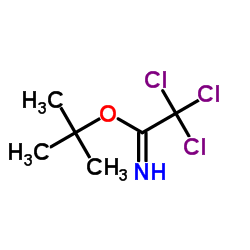 |
tert-Butyl 2,2,2-trichloroacetimidate
CAS:98946-18-0 |
C6H10Cl3NO |
|
Palmitoylation of plakophilin is required for desmosome asse...
2014-09-01 [J. Cell Sci. 127(Pt 17) , 3782-93, (2014)] |
|
Biocompatible click chemistry enabled compartment-specific p...
2014-01-01 [Nat. Commun. 5 , 4981, (2014)] |
|
A. Armstrong et al.
[Tetrahedron Lett. 29 , 2483, (1988)] |
|
[J. Chem. Soc. Perkin Trans. I , 2247, (1985)] |
|
[Tetrahedron Lett. 29 , 2483, (1988)] |