Journal of Organic Chemistry
2003-04-18
Reaction of some strong N-bases with chloropentafluorobenzene in the presence of water molecules.
Błazej Gierczyk, Grzegorz Schroeder, Bogumił Brzezinski
Index: J. Org. Chem. 68(8) , 3139-44, (2003)
Full Text: HTML
Abstract
Products of reactions between chloropentafluorobenzene and strong N-bases (DBN, DBU, TBD, and MTBD) in polar aprotic solvents and in the presence of water were isolated and identified by analytical and spectroscopic methods. The products of these dehydrohalogenation reactions are apropriate lactams, and if the TBD N-base is used, a condensation occurs with formation of benzimidazole derivative. All the products are not formed directly in the nucleophilic substitution by N-bases but after the hydrolyses of the N-bases with formation of amines known as hard nucleophiles.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
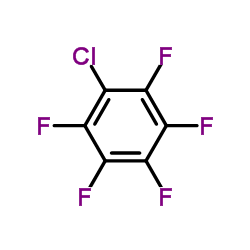 |
1-Chloro-2,3,4,5,6-pentafluorobenzene
CAS:344-07-0 |
C6ClF5 |
Related Articles:
More...
|
Gas chromatography with parallel hard and soft ionization ma...
2015-01-15 [Rapid Commun. Mass Spectrom. 29(1) , 91-9, (2014)] |
|
Oxidative dehalogenation of perhalogenated benzenes by cytoc...
2007-05-22 [Biochemistry 46(20) , 5924-40, (2007)] |
|
Catalytic and Noncatalytic Ammonolysis of Chloropentafluorob...
[Russ. J. Org. Chem. 37(3) , 404-9, (2001)] |
|
Synthesis of 1, 2 difluoro-1, 2-bis (pentafluorophenyl) dich...
[J. Fluor. Chem. 24(4) , 413-18, (1984)] |
|
5-Chloro-1-(difluorochloro)-2, 3, 4, 5, 6, 6-hexafluoro-1, 3...
[J. Fluor. Chem. 23(5) , 479, (1983)] |