Selective inhibition of cyclic AMP-dependent protein kinase by amphiphilic triterpenoids and related compounds.
B H Wang, G M Polya
Index: Phytochemistry 41(1) , 55-63, (1996)
Full Text: HTML
Abstract
A set of plant- and animal-derived amphiphilic triterpenoids have been shown to be potent and selective inhibitors of the catalytic subunit of rat liver cyclic AMP-dependent protein kinase (cAK). Thus plant-derived 18 alpha- and 18 beta-glycyrrhetinic acid, ursolic acid, oleanolic acid and betulin and animal-derived lithocholic acid, 5-cholenic acid and lithocholic acid methyl ester are inhibitors of cAK with IC50 values (concentrations for 50% inhibition) in the range 4-20 microM. These compounds are ineffective or relatively ineffective as inhibitors of various other eukaryote signal-regulated protein kinases namely wheat embryo Ca(2+)-dependent protein kinase (CDPK), avian calmodulin-dependent myosin light chain kinase (MLCK) and rat brain Ca(2+)- and phospholipid-dependent protein kinase C (PKC). These naturally occurring triterpenoids have a common structural motif involving polar residues located at opposite ends of an otherwise non-polar triterpenoid nucleus. A variety of triterpenoids not possessing this structural motif are relatively inactive as inhibitors of cAK and of CDPK, PKC and MLCK. The terpenoid amphiphilic compound crocetin is also a potent and relatively selective inhibitor of cAK (IC50 value for cAK 3.0 microM). 12-Hydroxystearic acid and 10-hydroxydecanoic acid do not inhibit CDPK, PKC or MLCK but are selective inhibitors of cAK (IC50 values 127 and 138 microM, respectively), consistent with a simple model for amphiphile inhibition of cAK involving two polar groups separated by a non-polar region. However, laurylgallate and 15-pentadecanolide are also potent and selective inhibitors of cAK (IC50 values 1.5 and 20 microM, respectively) although the structures of both of these compounds involve a large non-polar portion associated with only one polar region. Crocetin and the plant-derived amphiphilic triterpenoids described here are the most potent non-aromatic plant-derived inhibitors of cAK yet found.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
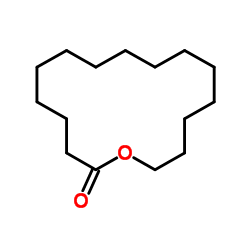 |
Cyclopentadecanolide
CAS:106-02-5 |
C15H28O2 |
|
A practical synthesis of long-chain iso-fatty acids (iso-C12...
2013-01-01 [Beilstein J. Org. Chem. 9 , 1807-12, (2013)] |
|
Effect of reaction temperature on the physicochemical proper...
[Polymer Bull. 72(3) , 1-12, (2015)] |
|
Anionic polymerization of pentadecanolide. A new route to po...
[Macromol. Chem. Phys. 197(9) , 1923-29, (1996)] |
|
A short synthesis of 15-pentadecanolide. Stanchev S, et al.
[Tetrahedron Lett. 34(38) , 6107-8, (1993)] |