Synthesis and characterization of [125I]N-(2-aminoethyl)-4-iodobenzamide as a selective monoamine oxidase B inhibitor.
H Rafii, S Chalon, J E Ombetta, Y Frangin, L Garreau, A M Dognon, I Lena, S Bodard, M P Vilar, J C Besnard
Index: Nucl. Med. Biol. 22(5) , 617-23, (1995)
Full Text: HTML
Abstract
We described the radiosynthesis of an analog of Ro 16-6491, [125I]N-(2-aminoethyl)-4-iodobenzamide, for SPECT exploration of the monoamine oxidase B (MAO-B) in human brain. The radiolabelling was carried out by nucleophilic exchange of the brominated precursor at solid-state phase in presence of ammonium sulphate. The radiochemical purity of radioiodinated product was higher than 95%. In comparison with Ro 16-6491, the in vitro studies showed a good selectivity of stable N-(2-aminoethyl)-4-iodobenzamide for MAO-B but a slightly lower affinity. Biodistribution studies in the rat showed a high and selective uptake of this compound in the pineal gland 1 h after i.v. injection. The cerebral uptake was low, but the coupling of [125I]N-(2-aminoethyl)-4-iodobenzamide with a lipophilic radical to enhance the passage through the blood-brain barrier can be envisaged.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
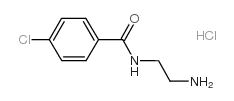 |
Ro 16-6491
CAS:94319-79-6 |
C9H12Cl2N2O |
|
The effects of phenelzine and other monoamine oxidase inhibi...
1995-02-01 [Br. J. Pharmacol. 114(4) , 837-45, (1995)] |
|
Overview of the present state of MAO inhibitors.
1987-01-01 [J. Neural Transm. Suppl. 23 , 103-19, (1987)] |
|
Aromatic L-amino acid decarboxylase (AAAD) activity in rhesu...
2005-04-01 [Synapse 56(1) , 54-6, (2005)] |
|
Interactions of the novel inhibitors of MAO-B Ro 19-6327 and...
1988-12-01 [Pharmacol. Res. Commun. 20 , 51, (1988)] |
|
[3H]Ro 16-6491, a selective probe for affinity labelling of ...
1988-04-01 [J. Neurochem. 50 , 1037, (1988)] |