|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![2H-Cyclopenta[b]pyridin-2-one,4-amino-1,5,6,7-tetrahydro-(9CI) Structure](https://image.chemsrc.com/caspic/202/63704-54-1.png)

![(5-acetamido-2-azabicyclo[4.3.0]nona-2,4,10-trien-3-yl) acetate Structure](https://image.chemsrc.com/caspic/395/5338-31-8.png)
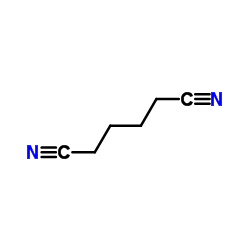
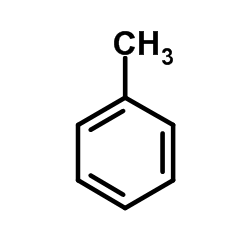
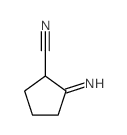
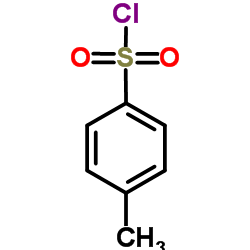
![N-(2-hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-4-yl)-4-methylbenzenesulfonamide Structure](https://image.chemsrc.com/caspic/306/5347-17-1.png)

![2H-Cyclopenta[b]pyridin-2-one,4-amino-3-bromo-1,5,6,7-tetrahydro Structure](https://image.chemsrc.com/caspic/225/6950-48-7.png)