Linker phosphoramidite reagents for the attachment of the first nucleoside to underivatized solid-phase supports.
Richard T Pon, Shuyuan Yu
Index: Nucleic Acids Res. 32(2) , 623-631, (2004)
Full Text: HTML
Abstract
New linker phosphoramidite reagents containing a cleavable 3'-ester linkage are used for attaching the first nucleoside to the surface of a solid- phase support. Inexpensive, underivatized amino supports, such as long chain alkylamine controlled-pore glass, can serve as universal supports. No modifications to phosphoramidite coupling conditions are required and, after synthesis, treatment with NH(4)OH releases the products with 3'-OH ends. No 3'-dephosphorylation is required. Phosphoramidite reagents containing a succinate and sulfonyl diethanol linkage between the nucleoside and phosphoramidite group are particularly advantageous and can be used to create both 3'-OH and 5'-phosphate ends on oligonucleotides. Reproducibility and quality of oligonucleotide synthesis is demonstrated for either column and 96-well plate formats on low-, medium- or high-loading CPG supports.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
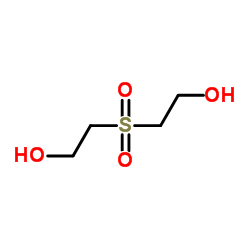 |
2,2'-Sulfonyldiethanol
CAS:2580-77-0 |
C4H10O4S |
|
Analysis of the sulphur mustard metabolites thiodiglycol and...
2007-01-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 845(1) , 114-20, (2007)] |
|
Analysis of urinary metabolites of sulfur mustard in two ind...
2008-01-01 [J. Anal. Toxicol. 32(1) , 10-6, (2008)] |
|
Methods for the analysis of thiodiglycol sulphoxide, a metab...
[J. Chromatogr. A. 558(2) , 393-404, (1991)] |
|
Improved methodology for the detection and quantitation of u...
1995-03-10 [J. Chromatogr. B, Biomed. Appl. 665(1) , 97-105, (1995)] |
|
Biological fate of sulphur mustard, 1,1'-thiobis(2-chloroeth...
1992-04-01 [Xenobiotica 22(4) , 405-18, (1992)] |