| Structure | Name/CAS No. | Articles |
|---|---|---|
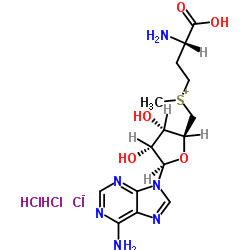 |
S-(5'-Adenosyl)-L-methionine chloride(SAM)
CAS:86867-01-8 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
S-(5'-Adenosyl)-L-methionine chloride(SAM)
CAS:86867-01-8 |