Microemulsion-mediated solvothermal synthesis and morphological evolution of MnCO3 nanocrystals.
Xinglong Wu, Minhua Caol, Hongyan Lü, Xiaoyan He, Changwen Hu
Index: J. Nanosci. Nanotechnol. 6(7) , 2123-8, (2006)
Full Text: HTML
Abstract
The morphology- and size-controlled synthesis of MnCO3 nanocrystals was successfully achieved by cationic surfactant-CTAB-microemulsion-mediated solvothermal method. Various comparison experiments with different reactant concentrations and molar ratios between water and CTAB, showed the evolvement law of the morphology and size of the as-synthesized MnCO3 nanocrystals. With slowly increasing the concentration of reactants and/or molar ratio between water and CTAB, the morphology of MnCO3 nanocrystals changed gradually from cube to parallelepiped, and then rhombohedron, whereas the size decreased a little. The effect of the experimental parameters on the shapes and sizes of samples, such as the source of carbonate salts, reaction time, and temperature, were also discussed. X-ray powder diffraction (XRD), transmission electron microscopy (TEM), selected area electron diffraction (SAED), and high-resolution transmission electron microscopy (HRTEM) were used to characterize the products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
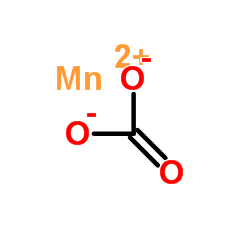 |
Manganese carbonate
CAS:598-62-9 |
CMnO3 |
|
Template-assisted assembly of the functionalized cubic and s...
2012-09-18 [Langmuir 28(37) , 13345-53, (2012)] |
|
Manganese sulfide formation via concomitant microbial mangan...
2011-12-01 [Environ. Microbiol. 13(12) , 3275-88, (2011)] |
|
Subcellular and gel chromatographic distribution of manganes...
1993-03-01 [Toxicol. Lett. 66(3) , 287-94, (1993)] |
|
Preclinical evaluation of manganese carbonate particles for ...
1995-02-01 [Acad. Radiol. 2(2) , 140-7, (1995)] |
|
Biomimetic synthesis of metal ion-doped hierarchical crystal...
2010-04-01 [Chem. Asian J. 5(4) , 792-8, (2010)] |