Acute renal and hepatic effects induced by 3-haloanilines in the Fischer 344 rat.
G O Rankin, M A Valentovic, D W Nicoll, J G Ball, D K Anestis, P I Brown, J L Hubbard
Index: J. Appl. Toxicol. 15(2) , 139-46, (1995)
Full Text: HTML
Abstract
Haloanilines are commonly used as chemical intermediates in the manufacture of a wide range of products. The purpose of this study was to examine the in vivo nephrotoxic and hepatotoxic potentials of the 3-haloanilines. The in vitro effects of the 3-haloanilines on renal function were also examined. In the in vivo experiments, male Fischer 344 rats (four rats/group) were administered a single intraperitoneal (i.p.) injection of an aniline hydrochloride (1.0 or 1.25 mmol kg-1) or vehicle. Renal and hepatic function were monitored at 24 and/or 48 h post-treatment. None of the 3-haloanilines were potent nephrotoxicants at either dose level. The greatest effects on renal function were observed following administration of 3-chloroaniline at a dose of 1.25 mmol kg-1 (oliguria, glucosuria, hematuria, decreased p-aminohippurate accumulation by renal cortical slices and increased blood urea nitrogen concentration). 3-Chloroaniline also was the only aniline compound to increase plasma ALT/GPT activity at 48 h. In the in vitro experiments, the ability of an aniline (10(-5) - 10(-3) M) to decrease organic ion accumulation in renal cortical slices from untreated rats was examined. The decreasing order of in vitro nephrotoxic potential was 3-iodoaniline > 3-bromoaniline > 3-chloroaniline > aniline > 3-fluoroaniline. These results indicate that the 3-haloanilines are not potent nephrotoxicants or hepatotoxicants at sublethal doses. In addition, the reasons why the 3-haloanilines have different orders of nephrotoxic potential in vivo and in vitro are not clear at this time.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
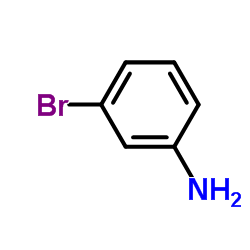 |
3-Bromoaniline
CAS:591-19-5 |
C6H6BrN |
|
Two-step hollow fiber-based, liquid-phase microextraction co...
2005-08-05 [J. Chromatogr. A. 1082(2) , 136-42, (2005)] |
|
m-[125I]iodoaniline: a useful reagent for radiolabeling biot...
1992-04-01 [Int. J. Rad. Appl. Instrum. B 19(3) , 297-301, (1992)] |
|
Cobb JM, et al.
[Tetrahedron Lett. 40(5) , 1045-1048, (1999)] |
|
A general palladium-catalyzed carbonylative synthesis of 2-a...
2012-10-01 [Chemistry 18(40) , 12599-602, (2012)] |