Synthesis of new β-1-C-alkylated imino-l-iditols: A comparative study of their activity as β-glucocerebrosidase inhibitors
Wojciech Schönemann, Estelle Gallienne, Philippe Compain, Kyoko Ikeda, Naoki Asano, Olivier R. Martin
Index: Bioorg. Med. Chem. 18(7) , 2645-50, (2010)
Full Text: HTML
Abstract
A short synthesis of new beta-1-C-alkyl-1,5-dideoxy-1,5-imino-l-iditols by means of the diastereoselective addition of Grignard reagents onto a glucopyranosylamine is described. These compounds were evaluated as beta-glucocerebrosidase inhibitors and their activity was compared with that of related iminosugar derivatives in the d-gluco and d-xylo series. The results allowed us to conclude on the influence of the hydroxymethyl moiety and of the piperidine-ring conformation on the inhibitory activity.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
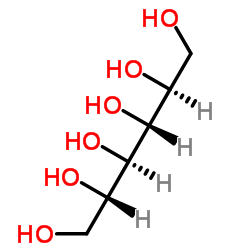 |
L-Iditol
CAS:488-45-9 |
C6H14O6 |
|
Possibility as monosaccharide laxative of rare sugar alcohol...
2009-05-01 [Yakugaku Zasshi 129 , 575-580, (2009)] |
|
Synthesis of D-mannitol and L-iditol derivatives as monomers...
2002-04-02 [Carbohydr. Res. 337(7) , 607-11, (2002)] |
|
Responses of the ant Lasius niger to various compounds perce...
2001-03-01 [Chem. Senses 26(3) , 231-7, (2001)] |
|
2-Ene-1,4-diols by dimerization of terminal epoxides using h...
2005-06-09 [Org. Lett. 7(12) , 2305-8, (2005)] |
|
Thermal mechanical analysis of frozen solutions of mannitol ...
1993-01-01 [J. Parenter. Sci. Technol. 47(3) , 119-23, (1993)] |