Xenobiotic triacylglycerol formation in isolated hepatocytes.
K G Moorhouse, P F Dodds, D H Hutson
Index: Biochem. Pharmacol. 41(8) , 1179-85, (1991)
Full Text: HTML
Abstract
The formation of neutral lipophilic metabolites from five xenobiotic carboxylic acids was studied in isolated rat hepatocytes. Oleic acid was used as a positive control. Rates of formation of lipids lay in the order: oleic acid greater than phytanic acid greater than ibuprofen greater than 3-phenoxybenzoic acid greater than indomethacin and 3-phenylbutanoic acid (rates were undetectable with the last two substrates). The process was saturable with the maximum rates at about 0.5 mM substrate concentration. Supplementation of the hepatocyte system with glycerol enhanced the yields of lipid products. The hepatocytes also effectively modelled the in vivo metabolism of ibuprofen, 3-phenoxybenzoic acid and 3-phenylbutanoic acid with oxidations and classical conjugation reactions predominating over xenobiotic lipid formation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
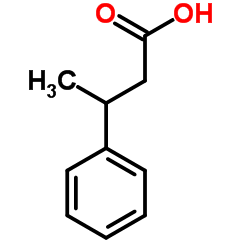 |
3-Phenylbutyric acid
CAS:4593-90-2 |
C10H12O2 |
|
Adsorption of small hydroxy acids on glass: a pitfall in qua...
1999-05-01 [J. Inherit. Metab. Dis. 22(3) , 293-6, (1999)] |
|
Induction of histone acetylation and growth regulation in er...
1999-01-01 [Anticancer Res. 19(3A) , 1971-6, (1999)] |
|
Enantioselective Metabolism of Chiral 3-Phenylbutyric Acid, ...
1996-03-01 [Appl. Environ. Microbiol. 62(3) , 749-55, (1996)] |
|
Oxidation of aliphatic, branched chain, and aromatic hydroca...
2010-06-01 [J. Basic Microbiol. 50(3) , 241-53, (2010)] |
|
Configurational analysis of chiral acids as O-trifluoroacety...
2000-09-08 [J. Chromatogr. A. 891(2) , 257-66, (2000)] |