Natural diversity to guide focused directed evolution.
Helge Jochens, Uwe T Bornscheuer
Index: ChemBioChem. 11(13) , 1861-6, (2010)
Full Text: HTML
Abstract
Simultaneous multiple site-saturation mutagenesis was performed at four active-site positions of an esterase from Pseudomonas fluorescens to improve its ability to convert 3-phenylbutyric acid esters (3-PBA) in an enantioselective manner. Based on an appropriate codon choice derived from a structural alignment of 1751 sequences of alpha/beta-hydrolase fold enzymes, only those amino acids were considered for library creation that appeared frequently in structurally equivalent positions. Thus, the number of mutants to be screened could be substantially reduced while the number of functionally intact variants was increased. Whereas the wild-type esterase showed only marginal activity and poor enantioselectivity (E(true)=3.2) towards 3-PBA-ethyl ester, a significant number of hits with improved rates (up to 240-fold) and enantioselectivities (up to E(true)=80) were identified in these "smart" libraries.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
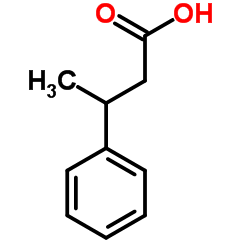 |
3-Phenylbutyric acid
CAS:4593-90-2 |
C10H12O2 |
|
Adsorption of small hydroxy acids on glass: a pitfall in qua...
1999-05-01 [J. Inherit. Metab. Dis. 22(3) , 293-6, (1999)] |
|
Induction of histone acetylation and growth regulation in er...
1999-01-01 [Anticancer Res. 19(3A) , 1971-6, (1999)] |
|
Enantioselective Metabolism of Chiral 3-Phenylbutyric Acid, ...
1996-03-01 [Appl. Environ. Microbiol. 62(3) , 749-55, (1996)] |
|
Oxidation of aliphatic, branched chain, and aromatic hydroca...
2010-06-01 [J. Basic Microbiol. 50(3) , 241-53, (2010)] |
|
Configurational analysis of chiral acids as O-trifluoroacety...
2000-09-08 [J. Chromatogr. A. 891(2) , 257-66, (2000)] |