Synthesis and preliminary biological evaluation of truncated zoanthenol analogues.
Go Hirai, Hiroki Oguri, Masahiko Hayashi, Koji Koyama, Yuuki Koizumi, Sameh M Moharram, Masahiro Hirama
Index: Bioorg. Med. Chem. Lett. 14 , 2647, (2004)
Full Text: HTML
Abstract
Zoanthamines are a family of marine alkaloids that have complex heptacyclic structures and are reported to be interleukin-6 modulators. While the structure of zoanthamines, especially the ABC-ring portion, is similar to that of steroids, the CDEFG-ring portion, composed of aminoacetal and lactone core, is a unique structural element. In this report, we designed and synthesized ABC-ring 6 and CDEFG-ring 7, which are truncated analogues of the northern and southern hemispheres of zoanthenol 5, respectively, and which incorporate all of the functionality of each hemisphere. A preliminary SAR study suggested that the hydrochloride of the CEFG-ring portion is an active pharmacophore for suppressing the growth of interleukin-6-dependent MH60 cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
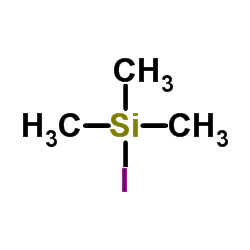 |
Trimethylsilyl iodide
CAS:16029-98-4 |
C3H9ISi |
|
Occurrence, removal, and fate of progestogens, androgens, es...
2014-11-01 [Environ. Sci. Pollut. Res. Int. 21(22) , 12898-908, (2014)] |
|
Validation of an enzyme immunoassay for the measurement of f...
2014-09-15 [Gen. Comp. Endocrinol. 206 , 166-77, (2014)] |
|
[Determination of anabolic steroid hormones in animal muscle...
2006-01-01 [Se Pu 24(1) , 19-22, (2006)] |
|
[Tetrahedron Lett. 35 , 5445, (1994)] |
|
[Tetrahedron Lett. 45 , 3607, (2004)] |