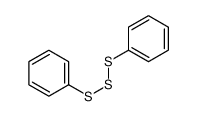
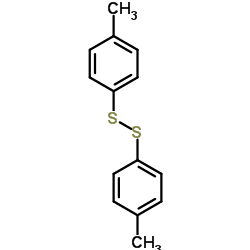
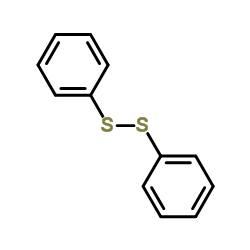
|
~% |
|
~59% |
|
~43% |
|
~95% |
|
~92% |
|
~79% |
|
~62% |
|
~82% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~% |
|
~0% |
|
~% |
|
~0% |
|
~82% |
![1-tert-butyl-4-[(4-tert-butylphenyl)trisulfanyl]benzene Structure](https://image.chemsrc.com/caspic/311/13846-49-6.png)
![N-[bis(diethylamino)phosphinothioyl]-N-ethylethanamine Structure](https://image.chemsrc.com/caspic/370/4154-77-2.png)
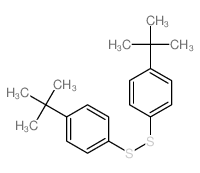
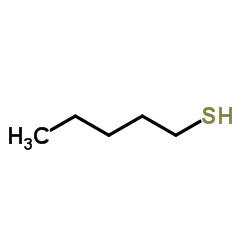
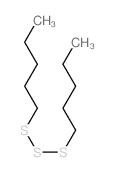
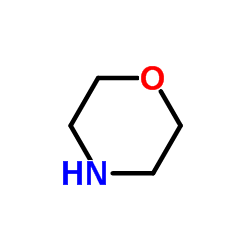
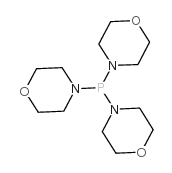
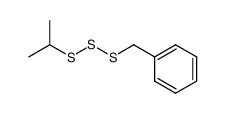

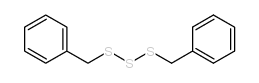
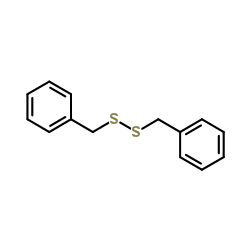
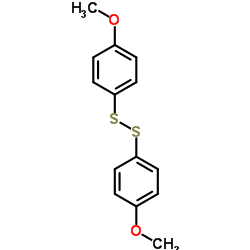
![1-methoxy-4-[(4-methoxyphenyl)trisulfanyl]benzene Structure](https://image.chemsrc.com/caspic/166/20057-92-5.png)