Two general routes to 1,4-disubstituted-2,3,4,5-tetrahydro- 1H-3-benzazepines.
S W Gerritz, J S Smith, S S Nanthakumar, D E Uehling, J E Cobb
Index: Org. Lett. 2(25) , 4099-102, (2000)
Full Text: HTML
Abstract
[structure] Two general routes to 1,4-disubstituted-2,3,4, 5-tetrahydro-1H-3-benzazepines are described. Both routes utilize an appropriately functionalized phenethylamino alcohol as the penultimate intermediate: the first route makes use of the reductive amination of a benzyl alkyl ketone with alpha-(aminomethyl)benzyl alcohol, while the second route utilizes the addition of a Grignard reagent to the oxazolidine derived from a substitued phenylacetaldehyde and alpha-(methylaminomethyl)benzyl alcohol. In all cases studied, the cis-1,4-disubstituted-2,3,4, 5-tetrahydro-1H-3-benzazepine was obtained as the major product.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
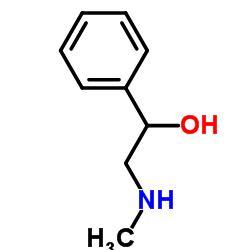 |
DL-ALPHA-(METHYLAMINOMETHYL)BENZYL ALCOHOL
CAS:6589-55-5 |
C9H13NO |
|
MMA/MPEOMA/VSA copolymer as a novel blood-compatible materia...
2004-02-01 [J. Mater. Sci. Mater. Med. 15(2) , 155-9, (2004)] |
|
Phe310 in transmembrane VI of the alpha1B-adrenergic recepto...
1999-06-04 [J. Biol. Chem. 274(23) , 16320-30, (1999)] |
|
Characterization of N-methylphenylethylamine and N-methylphe...
1980-10-01 [Biochem. Pharmacol. 29(19) , 2663-7, (1980)] |
|
Elimination of ephedrines in urine following multiple dosing...
2004-01-01 [Br. J. Clin. Pharmacol. 57(1) , 62-7, (2004)] |
|
The vasoactive potential of halostachine, an alkaloid of tal...
1983-12-01 [Vet. Hum. Toxicol. 25(6) , 408-11, (1983)] |