Molecules
2005-01-01
Improved synthesis of beta-D-6-methylpurine riboside and antitumor effects of the beta-D- and alpha-D-anomers.
Canio J Marasco, Paula J Pera, Arthur J Spiess, Ralph Bernacki, Janice R Sufrin
Index: Molecules 10(8) , 1015-20, (2005)
Full Text: HTML
Abstract
6-Methylpurine-beta-D-riboside (beta-D-MPR) has been synthesized by coupling 6-methylpurine and 1-O-acetyl-2,3,5-tri-O-benzoyl-D-ribose using conditions that produce the beta-D-anomer exclusively. The in vitro antitumor effects of beta-D-MPR and 6-methyl-purine-alpha-D-riboside (alpha-D-MPR) in five human tumor cell lines showed that beta-D-MPR was highly active (IC(50) values ranging from 6 to 34 nM). alpha-D-MPR, although less active than beta-D-MPR, also exhibited significant antitumor effects (IC50 values ranging from 1.47 to 4.83 microM).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
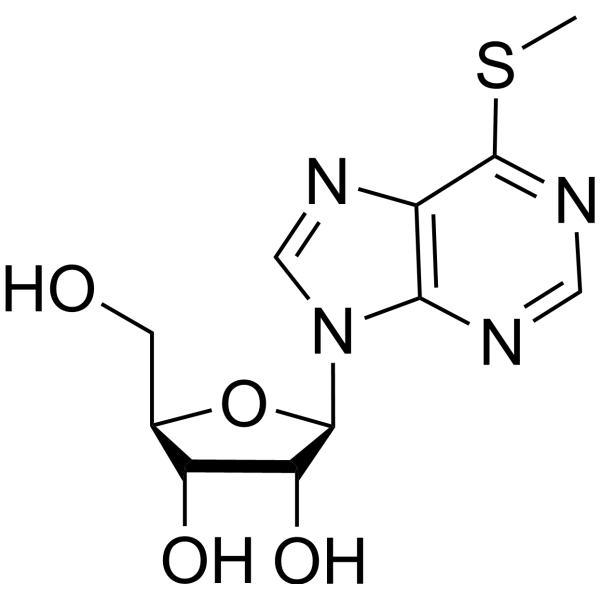 |
Inosine,6-S-methyl-6-thio
CAS:342-69-8 |
C11H14N4O4S |
Related Articles:
More...
|
The role of inosine-5'-monophosphate dehydrogenase in thiopu...
2011-04-01 [Ther. Drug Monit. 33 , 200-208, (2011)] |
|
Methylthioadenosine deaminase in an alternative quorum sensi...
2012-11-13 [Biochemistry 51(45) , 9094-103, (2012)] |
|
A prospective, open-label trial of 6-thioguanine in patients...
2005-10-01 [Scand. J. Gastroenterol. 40(10) , 1205-13, (2005)] |
|
Thiopurines inhibit bovine viral diarrhea virus production i...
2008-04-01 [J. Gen. Virol. 89 , 1000-1009, (2008)] |
|
Methylthioinosine phosphorylase from Pseudomonas aeruginosa....
2011-02-22 [Biochemistry 50(7) , 1247-54, (2011)] |