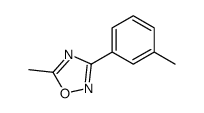
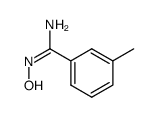
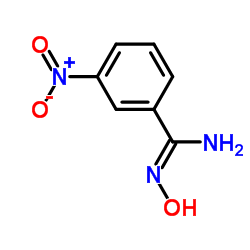
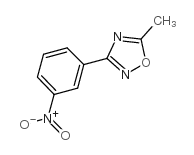
|
~% |
|
~92% |
|
~% |
|
~74% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |

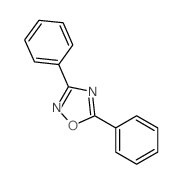
![[[amino-(4-methylphenyl)methylidene]amino] acetate Structure](https://image.chemsrc.com/caspic/040/88303-28-0.png)
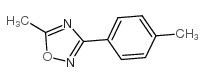

![[[amino-(2-methylphenyl)methylidene]amino] acetate Structure](https://image.chemsrc.com/caspic/126/88303-26-8.png)
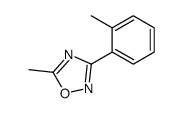
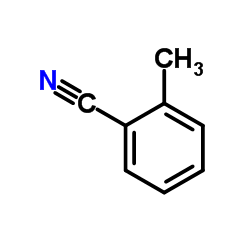

![[[amino-(3-methylphenyl)methylidene]amino] acetate Structure](https://image.chemsrc.com/caspic/088/88303-27-9.png)