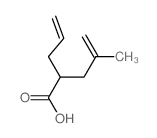
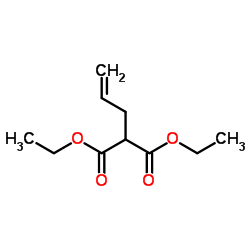
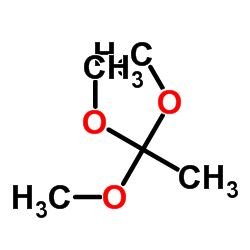
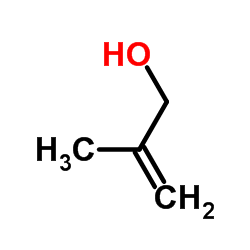
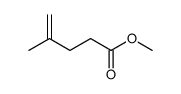
|
~% |
|
~% |
|
~% |
|
~74% |
|
~99% |
![2-[3,5-bis(4-methoxyphenyl)-4,5-dihydropyrazol-1-yl]-4-(4-fluorophenyl)-1,3-thiazole hydrobromide Structure](https://image.chemsrc.com/caspic/118/5309-50-2.png)