A further study on the combined use of internal standard and isotope-labeled derivatization reagent for expansion of linear dynamic ranges in liquid chromatography-electrospray mass spectrometry.
Yuhki Tsukamoto, Tomofumi Santa, Hiroo Yoshida, Hiroshi Miyano, Takeshi Fukushima, Kazuo Hirayama, Kazuhiro Imai, Takashi Funatsu
Index: Biomed. Chromatogr. 20(10) , 1049-55, (2006)
Full Text: HTML
Abstract
The combined use of a so-called internal standard and the isotope-labeled derivatization reagent for the quantification of analytes for liquid chromatography-mass spectrometry (LC/MS) was further studied. The sample solution (containing the analytes and an internal standard) was derivatized with the light form of the derivatization reagent, 7-(N,N-dimethylaminosulfonyl)-4-(aminoethyl)piperazino-2,1,3-benzoxadiazole (DBD-PZ-NH(2)) or 7-(N,N-dimethylaminosulfonyl)-4-piperazino-2,1,3-benzoxadiazole (DBD-PZ). A standard solution of the analytes (containing an internal standard) was derivatized with the isotope (d(6))-labeled derivatization reagent, DBD-PZ-NH(2) (D) or DBD-PZ (D), and served as the isotope-labeled internal standards. The peak heights of the targeted analytes derivatives in the sample solution were corrected using those of the internal standard and the heavy form derivatives of the standards, and the calibration curves were constructed. The curve bending of the calibration curves caused by the ion suppression at the ion source was suppressed and the linear dynamic ranges of the calibration curves were expanded. The derivatives of DBD-PZ-NH(2) were about 10 times more sensitively detected than those of DBD-PZ derivatives and, therefore, DBD-PZ-NH(2) might be suitable for sensitive detection.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
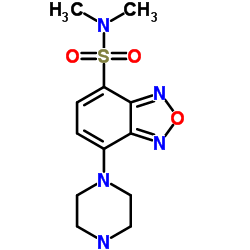 |
dbd-pz
CAS:139332-64-2 |
C12H17N5O3S |
|
Sensitive fluorometric detection of prostaglandins by high p...
1992-01-01 [Biomed. Chromatogr. 6 , 143, (1992)] |
|
Enantiomeric determination of D- and L-lactate in rat serum ...
1999-05-01 [Anal. Biochem. 269(2) , 379-85, (1999)] |
|
Development of an HPLC-fluorescence determination method for...
2005-12-01 [Biomed. Chromatogr. 19(10) , 788-95, (2005)] |
|
Fluorimetric determination of D- and L-lactate derivatized w...
1999-10-01 [Biomed. Chromatogr. 13(6) , 418-24, (1999)] |
|
Sensitive analytical method for the novel H1-receptor antago...
1996-12-13 [J. Chromatogr. B, Biomed. Appl. 687(2) , 419-25, (1996)] |