| Structure | Name/CAS No. | Articles |
|---|---|---|
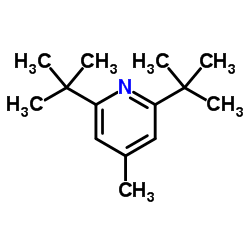 |
2,6-Di-tert-butyl-4-methylpyridine
CAS:38222-83-2 |
Katsumi Kobayashi, Mieko Arisawa, Masahiko Yamaguchi
Index: J. Am. Chem. Soc. 124(29) , 8528-9, (2002)
Full Text: HTML
Phenols are ethynylated at the ortho position with silylated chloroethyne in the presence of a catalytic amount of GaCl3 and lithium phenoxide. The lithium salt is essential for the catalysis, and addition of 2,6-di(tert-butyl)-4-methylpyridine inhibits desilylation and hydration of the products. The reaction can be applied to various substituted phenols giving the ortho-ethynylated products in high yields, and the turnover numbers based on GaCl3 are between 8 and 10. The reaction mechanism involves addition of in situ formed phenoxygallium to the haloethyne followed by the elimination of GaCl3.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
2,6-Di-tert-butyl-4-methylpyridine
CAS:38222-83-2 |
C14H23N |
|
Cyclization reactions of homopropargyl azide derivatives cat...
2006-11-09 [Org. Lett. 8(23) , 5349-52, (2006)] |
|
2-Methoxybenzoyl phosphate: a new substrate for continuous f...
1995-01-15 [J. Org. Chem. 65(3) , 801-5, (2000)] |
|
Silylium ion-promoted dehydrogenative cyclization: synthesis...
2014-06-25 [Chem. Commun. (Camb.) , (2014)] |
|
[Synthesis , 283, (1980)] |
|
[Organic Synth. 68 , 138, (1990)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved