Evidence of preorganization in quinonoid intermediate formation from L-Trp in H463F mutant Escherichia coli tryptophan indole-lyase from effects of pressure and pH.
Robert S Phillips, Ukoha Kalu, Sam Hay
Index: Biochemistry 51(33) , 6527-33, (2012)
Full Text: HTML
Abstract
The effects of pH and hydrostatic pressure on the reaction of H463F tryptophan indole-lyase (TIL) have been evaluated. The mutant TIL shows very low activity for elimination of indole but is still competent to form a quinonoid intermediate from l-tryptophan [Phillips, R. S., Johnson, N., and Kamath, A. V. (2002) Biochemistry 41, 4012-4019]. Stopped-flow measurements show that the formation of the quinonoid intermediate at 505 nm is affected by pH, with a bell-shaped dependence for the forward rate constant, k(f), and dependence on a single basic group for the reverse rate constant, k(r), with the following values: pK(a1) = 8.14 ± 0.15, pK(a2) = 7.54 ± 0.15, k(f,min) = 18.1 ± 1.3 s(-1), k(f,max) = 179 ± 46.3 s(-1), k(r,min) = 11.4 ± 1.2 s(-1), and k(r,max) = 33 ± 1.6 s(-1). The pH effects may be due to ionization of Tyr74 as the base and Cys298 as the acid influencing the rate constant for deprotonation. High-pressure stopped-flow measurements were performed at pH 8, which is the optimum for the forward reaction. The rate constants show an increase with pressure up to 100 MPa and a subsequent decrease above 100 MPa. Fitting the pressure data gives the following values: k(f,0) = 15.4 ± 0.8 s(-1), ΔV(‡) = -29.4 ± 2.9 cm(3) mol(-1), and Δβ(‡) = -0.23 ± 0.03 cm(3) mol(-1) MPa(-1) for the forward reaction, and k(r,0) = 20.7 ± 0.8 s(-1), ΔV(‡) = -9.6 ± 2.3 cm(3) mol(-1), and Δβ(‡) = -0.05 ± 0.02 cm(3) mol(-1) MPa(-1) for the reverse reaction. The primary kinetic isotope effect on quinonoid intermediate formation at pH 8 is small (~2) and is not significantly pressure-dependent, suggesting that the effect of pressure on k(f) may be due to perturbation of an active site preorganization step. The negative activation volume is also consistent with preorganization of the ES complex prior to quinonoid intermediate formation, and the negative compressibility may be due to the effect of pressure on the enzyme conformation. These results support the conclusion that the preorganization of the H463F TIL Trp complex, which is probably dominated by motion of the l-Trp indole moiety of the aldimine complex, contributes to quinonoid intermediate formation.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
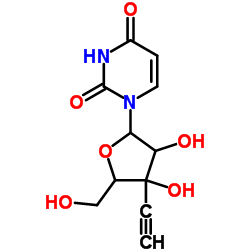 |
Tryptophanase
CAS:9024-00-4 |
C11H12N2O6 |
|
Inhibition of Escherichia coli tryptophan indole-lyase by tr...
2014-10-15 [Arch. Biochem. Biophys. 560 , 20-6, (2014)] |
|
Reaction pathway of tryptophanase-catalyzed L-tryptophan syn...
2011-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(29) , 3289-95, (2011)] |
|
Indole production by the tryptophanase TnaA in Escherichia c...
2013-02-01 [Microbiology 159(Pt 2) , 402-10, (2013)] |
|
Properties of tryptophanase from Escherichia coli.
1962-12-04 [Biochim. Biophys. Acta 65 , 233-44, (1962)] |
|
Antibiotics promoting oxidative stress inhibit formation ofE...
2010-01-01 [Res. Microbiol. 161(10) , 847-53, (2010)] |