Effects of bromohomoibotenate on metabotropic glutamate receptors.
C Thomsen, A Bau, P Faarup, C Foged, A Kanstrup, P D Suzdak
Index: Neuroreport 5(18) , 2417-20, (1994)
Full Text: HTML
Abstract
(S)-Bromohomoibotenic acid [(S)-BrHIbo] stereoselectively antagonized glutamate-stimulated phosphoinositide (PI) hydrolysis in baby hamster kidney (BHK) cells expressing mGluR1a in a competitive manner with an IC50 of 250 microM. However, (S)-BrHIbo did not inhibit (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid [(1S,3R)-ACPD]-induced PI hydrolysis in rat hippocampal slices (S)- or (R)-BrHIbo did not show any effects on forskolin-stimulated cAMP-formation in BHK cells expressing mGluR2 or mGluR4 but did displace [3H]2-amino-4-phosphonobutyrate ([3H]AP4) binding from rat corticalmembranes with high affinities (IC50 = 1.0 microM and 1.1 microM, respectively). These data suggest that (S)-BrHIbo may interest with multiple PI-coupled glutamate receptors, however, at concentrations that are several fold higher than for displacement of [3H]AP4 binding from rat cortical membranes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
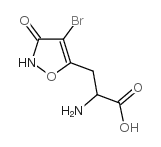 |
(RS)-1-(3-FLUOROPHENYL)ETHYLAMINE
CAS:71366-32-0 |
C6H7BrN2O4 |
|
Turning behaviour and catalepsy after injection of excitator...
1981-05-29 [Neurosci. Lett. 23 , 337, (1981)] |
|
Excitatory amino acid agonists. Enzymic resolution, X-ray st...
1989-10-01 [J. Med. Chem. 32(10) , 2254-60, (1989)] |
|
Structural determinants of agonist-specific kinetics at the ...
2005-08-23 [Proc. Natl. Acad. Sci. U. S. A. 102(34) , 12053-8, (2005)] |
|
Identification of amino acid residues in GluR1 responsible f...
2001-05-01 [J. Neurosci. 21(9) , 3052-62, (2001)] |
|
4-Methylhomoibotenic acid activates a novel metabotropic glu...
1997-11-01 [J. Pharmacol. Exp. Ther. 283(2) , 742-9, (1997)] |