Controlling phase separation and optical properties in conjugated polymers through selenophene-thiophene copolymerization.
Jon Hollinger, Ashlee A Jahnke, Neil Coombs, Dwight S Seferos
Index: J. Am. Chem. Soc. 132(25) , 8546-7, (2010)
Full Text: HTML
Abstract
Selenophene-thiophene block copolymers were synthesized and studied. The properties of these novel block copolymers are distinct from those of statistical copolymers prepared from the same monomers with a similar composition. Specifically, the block copolymers exhibit broad and red-shifted absorbance features and phase-separated domains in the solid state. Scanning transmission electron microscopy and topographic elemental mapping confirmed that the domains are either rich in selenophene or thiophene, indicating that the blocks of distinct heterocycles preferentially associate with one another in the solid state. This preference is surprising in view of the chemical similarities between repeat units. The overall results demonstrate a phase separation that is controlled by elemental differences. As a result of this phase separation, these novel conjugated block copolymers should find utility in a variety of studies and optoelectronics uses.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
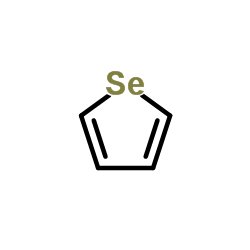 |
Selenophene
CAS:288-05-1 |
C4H4Se |
|
General synthesis of thiophene and selenophene-based heteroa...
2005-11-10 [Org. Lett. 7(23) , 5301-4, (2005)] |
|
[J. Electrochem. Soc. 137 , 1827, (1990)] |
|
Dye-sensitized solar cells based on organic sensitizers with...
[J. Phys. Chem. C Nanomater. Interfaces 113(17) , 7469-79, (2009)] |
|
Low band gap selenophene-diketopyrrolopyrrole polymers exhib...
[Chem. Sci. 3(1) , 181-85, (2012)] |
|
Electron transmission spectra of selenophene and tellurophen...
[Chem. Phys. 88(3) , 181-85, (1984)] |