|
~80% |
|
~% |
|
~48% |
|
~25% |
|
~81% |
|
~66% |
|
~17% |
|
~16% |
|
~22% |
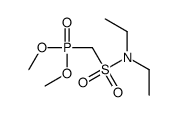




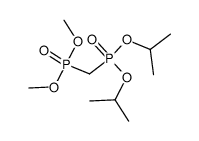
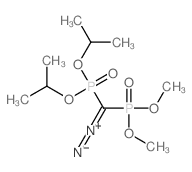
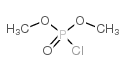
![dimethyl [[diethylamino)sulfonyl]methoxymethyl]phosphonate Structure](https://image.chemsrc.com/caspic/275/80721-52-4.png)


![dimethyl [[(diethylamino)sulfonyl]ethoxymethyl]phosphonate Structure](https://image.chemsrc.com/caspic/237/80721-53-5.png)