Primary structure of a zinc protease from Bacillus mesentericus strain 76.
S Stoeva, T Kleinschmidt, B Mesrob, G Braunitzer
Index: Biochemistry 29(2) , 527-34, (1990)
Full Text: HTML
Abstract
The amino acid sequence of the neutral zinc protease from Bacillus mesentericus strain 76 (MCP 76) has been determined by using peptides derived from digests with trypsin, chymotrypsin, and cyanogen bromide and from cleavage with o-iodosobenzoic acid. The peptides were purified by means of gel filtration and reversed-phase high-performance liquid chromatography and analyzed by automatic sequencing. The protein contains 300 amino acid residues. It proved to be identical with the neutral protease deduced from the DNA precursor sequence of Bacillus subtilis. The residues for zinc and substrate binding are conserved, whereas the number of calcium binding sites is reduced compared to thermolysin. A classification of the neutral zinc protease is discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
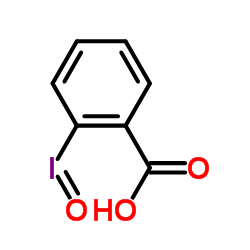 |
2-Iodosylbenzoic acid
CAS:304-91-6 |
C7H5IO3 |
|
Reduction of nitroaromatic compounds by anaerobic bacteria i...
1991-04-01 [Appl. Environ. Microbiol. 57(4) , 962-8, (1991)] |
|
Rat liver 6-phosphofructo-2-kinase/fructose-2,6-bisphosphata...
1987-08-25 [J. Biol. Chem. 262(24) , 11714-20, (1987)] |
|
Epitopes of Escherichia coli ribosomal protein S13.
1989-12-01 [J. Protein Chem. 8(6) , 701-17, (1989)] |
|
Influence of the polymer-micelle interaction on micelle-subs...
2010-03-01 [J. Pharm. Sci. 99(3) , 1440-51, (2010)] |
|
Tryptic digestion of bovine secretory IgA at elevated temper...
1989-01-01 [Int. J. Biochem. 21(5) , 549-54, (1989)] |