Identification of arg-30 as the essential residue for the enzymatic activity of Taiwan cobra phospholipase A2.
L S Chang, S R Lin, C C Chang
Index: J. Biochem. 124(4) , 764-8, (1998)
Full Text: HTML
Abstract
Taiwan cobra (Naja naja atra) phospholipase A2 (PLA2) was inactivated by arginine-specific reagents, phenylglyoxal and 1, 2-cyclohexanedione. Kinetic analyses of the modification reaction revealed that the inactivation of PLA2 followed pseudo-first-order kinetics and the loss of activity was correlated with the incorporation of one molecule of modification reagent per PLA2 molecule. This was confirmed by the results of amino acid composition determination, that showed that a marked decrease in enzymatic activity was associated with the modification of one arginine residue. Tryptic cleavage of the modified protein and microsequencing revealed that Arg-30 was the functionally essential residue. The incorporation of a modifier into the PLA2 did not significantly affect the secondary structure of the enzyme, as revealed by the CD spectrum, and Ca2+-binding of the modified PLA2 was unaffected. Nevertheless, the nonpolarity of the active site of PLA2 markedly decreased with the arginine modification, as evidenced by the decreases in the enhancement of Trp and 8-anilinonaphthalene sulfonate fluorescence. These results, together with those of X-ray crystallographic analysis of N. naja atra PLA2 [Scott et al. (1990) Science 250, 1541-1546], demonstrate that Arg-30 is one of the residues involved in the interfacial binding of a PLA2 molecule with its substrate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
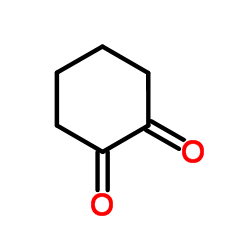 |
1,2-Cyclohexanedione
CAS:765-87-7 |
C6H8O2 |
|
Chemical modification of cationic groups in the polypeptide ...
1995-02-01 [Toxicon 33(2) , 187-99, (1995)] |
|
Modification of arginine-198 in sarcoplasmic reticulum Ca2+-...
1997-08-22 [J. Biol. Chem. 272(34) , 21142-50, (1997)] |
|
Evidence for an essential arginine in the flavoprotein nitro...
2001-01-01 [J. Enzym. Inhib. 16(2) , 157-63, (2001)] |
|
Modification of arginine residues in ovine prolactin by 1,2-...
1994-07-01 [Int. J. Pept. Protein Res. 44(1) , 31-5, (1994)] |
|
Identification of arginyl residues located at the ATP bindin...
1996-11-15 [J. Biol. Chem. 271(46) , 28933-41, (1996)] |