Journal of Organic Chemistry
2009-11-06
Rhodium-catalyzed synthesis of 3-hydroxy-beta-lactams via oxonium ylide generation: three-component reaction between azetidine-2,3-diones, ethyl diazoacetate, and alcohols.
Benito Alcaide, Pedro Almendros, Cristina Aragoncillo, Ricardo Callejo, M Pilar Ruiz, M Rosario Torres
Index: J. Org. Chem. 74(21) , 8421-4, (2009)
Full Text: HTML
Abstract
3-Substituted-3-hydroxy-beta-lactams, with two new adjacent stereogenic centers, have been prepared in a single step by a rhodium-catalyzed, three-component reaction between azetidine-2,3-diones, ethyl diazoacetate, and alcohols. Good to moderate stereoselectivity was obtained depending on the alcohol used. The stereochemistry of the new centers has been undoubtedly assigned by single crystal X-ray diffraction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
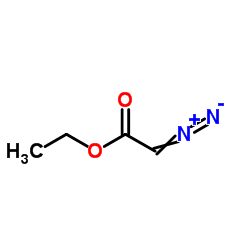 |
EDA
CAS:623-73-4 |
C4H6N2O2 |
Related Articles:
More...
|
Design of a Heterogeneous Catalyst Based on Cellulose Nanocr...
2015-08-24 [Chemistry 21 , 12414-20, (2015)] |
|
Silver-catalyzed vinylogous fluorination of vinyl diazoaceta...
2013-12-20 [Org. Lett. 15(24) , 6152-4, (2013)] |
|
An efficient and convenient synthesis of ethyl 1-(4-methoxyp...
2010-02-01 [Chem. Asian J. 5(2) , 328-33, (2010)] |
|
Component match in rhodium catalyzed three-component reactio...
2009-12-07 [Org. Biomol. Chem. 7(23) , 5028-33, (2009)] |
|
Palladium-catalyzed cross-coupling of aryl or vinyl iodides ...
2007-07-18 [J. Am. Chem. Soc. 129(28) , 8708-9, (2007)] |