Formal aromatic C-H insertion for stereoselective isoquinolinone synthesis and studies on mechanistic insights into the C-C bond formation.
Chan Pil Park, Advait Nagle, Cheol Hwan Yoon, Chiliu Chen, Kyung Woon Jung
Index: J. Org. Chem. 74(16) , 6231-6, (2009)
Full Text: HTML
Abstract
Formal aromatic C-H insertion of rhodium(II) carbenoid was intensively investigated to develop a new methodology and probe its mechanism. Contrasting with the previously proposed direct C-H insertion, the mechanism was revealed to be electrophilic aromatic substitution, which was supported by substituent effects on the aromatic ring and a secondary deuterium kinetic isotope effect. Various isoquinolinones were synthesized intramolecularly via six-membered ring formation with high regio- and diastereoselectivity, while averting the common Buchner-type reaction. Intermolecularly, dirhodium catalyzed formal aromatic C-H insertion on electron-rich aromatics was also achieved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
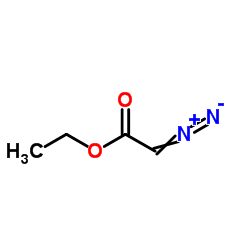 |
EDA
CAS:623-73-4 |
C4H6N2O2 |
|
Design of a Heterogeneous Catalyst Based on Cellulose Nanocr...
2015-08-24 [Chemistry 21 , 12414-20, (2015)] |
|
Silver-catalyzed vinylogous fluorination of vinyl diazoaceta...
2013-12-20 [Org. Lett. 15(24) , 6152-4, (2013)] |
|
An efficient and convenient synthesis of ethyl 1-(4-methoxyp...
2010-02-01 [Chem. Asian J. 5(2) , 328-33, (2010)] |
|
Component match in rhodium catalyzed three-component reactio...
2009-12-07 [Org. Biomol. Chem. 7(23) , 5028-33, (2009)] |
|
Palladium-catalyzed cross-coupling of aryl or vinyl iodides ...
2007-07-18 [J. Am. Chem. Soc. 129(28) , 8708-9, (2007)] |