Journal of Organic Chemistry
2004-04-02
Vitamin B12 derivatives as natural asymmetric catalysts: enantioselective cyclopropanation of alkenes.
Ying Chen, X Peter Zhang
Index: J. Org. Chem. 69(7) , 2431-5, (2004)
Full Text: HTML
Abstract
Vitamin B(12) derivatives were found for the first time to be general and efficient catalysts for asymmetric cyclopropanation of alkenes with ethyl diazoacetate (EDA). Among several common derivatives, aquocobalamin (B(12a)) was shown to be the most effective catalyst for a variety of alkenes, providing cis-dominant cyclopropanes in excellent yields and moderate enantioselectivity. Reactivity studies under different conditions suggest that the active species in the proposed catalytic cycle is the base-on cob(II)alamin (B(12r)) that is generated possibly via in situ reduction of B(12a) by EDA.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
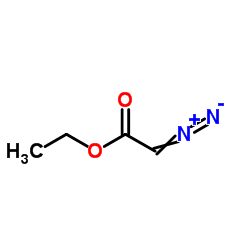 |
EDA
CAS:623-73-4 |
C4H6N2O2 |
Related Articles:
More...
|
Design of a Heterogeneous Catalyst Based on Cellulose Nanocr...
2015-08-24 [Chemistry 21 , 12414-20, (2015)] |
|
Silver-catalyzed vinylogous fluorination of vinyl diazoaceta...
2013-12-20 [Org. Lett. 15(24) , 6152-4, (2013)] |
|
An efficient and convenient synthesis of ethyl 1-(4-methoxyp...
2010-02-01 [Chem. Asian J. 5(2) , 328-33, (2010)] |
|
Component match in rhodium catalyzed three-component reactio...
2009-12-07 [Org. Biomol. Chem. 7(23) , 5028-33, (2009)] |
|
Palladium-catalyzed cross-coupling of aryl or vinyl iodides ...
2007-07-18 [J. Am. Chem. Soc. 129(28) , 8708-9, (2007)] |