| Structure | Name/CAS No. | Articles |
|---|---|---|
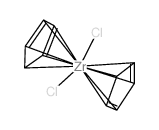 |
Bis(cyclopentadienyl)zirconium dichloride
CAS:1291-32-3 |
Leo A Paquette, Yunlong Zhang
Index: Org. Lett. 7(3) , 511-3, (2005)
Full Text: HTML
[reaction: see text] A synthetic route to select cyclooctane-1,2,3-triols and 1,2,3,4,5-pentaols has been defined. The starting materials are d-glucose or d-arabinose, and the key steps consist of a zirconocene-promoted ring contraction, a [3,3] sigmatropic rearrangement, and more extended functionalization of the resulting cyclooctadienone.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Bis(cyclopentadienyl)zirconium dichloride
CAS:1291-32-3 |
C10H2Cl2Zr |
|
Unusual temperature effects in propene polymerization using ...
2003-02-15 [ChemPhysChem 6(9) , 1929-33, (2005)] |
|
Functionalised cyclopentadienyl zirconium compounds as poten...
2008-10-21 [Dalton Trans. (39) , 5293-5, (2008)] |
|
Formal total synthesis of (+/-)-estrone and zirconocene-prom...
2008-08-15 [J. Org. Chem. 73(16) , 6202-6, (2008)] |
|
In pursuit of pestalotiopsin a via zirconocene-mediated ring...
2006-05-25 [Org. Lett. 8(11) , 2429-31, (2006)] |
|
A selective synthesis of hydroxyborate anions as novel ancho...
2008-06-07 [Dalton Trans. (21) , 2866-70, (2008)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved