| Structure | Name/CAS No. | Articles |
|---|---|---|
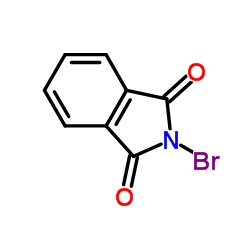 |
2-Bromo-1H-isoindole-1,3(2H)-dione
CAS:2439-85-2 |
Feng Chen, Chong Kiat Tan, Ying-Yeung Yeung
Index: J. Am. Chem. Soc. 135(4) , 1232-5, (2013)
Full Text: HTML
A catalytic asymmetric bromocyclization of trisubstituted olefinic amides that uses a C(2)-symmetric mannitol-derived cyclic selenium catalyst and a stoichiometric amount of N-bromophthalimide is reported. The resulting enantioenriched pyrrolidine products, which contain two stereogenic centers, can undergo rearrangement to yield 2,3-disubstituted piperidines with excellent diastereoselectivity and enantiospecificity.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
2-Bromo-1H-isoindole-1,3(2H)-dione
CAS:2439-85-2 |
C8H4BrNO2 |
|
Enantioselective synthesis of multisubstituted biaryl skelet...
2013-03-13 [J. Am. Chem. Soc. 135(10) , 3964-70, (2013)] |
|
Titrimetric determination of acetylenic hyponotics using org...
1988-04-22 [Pharm. Weekbl. Sci. 10(2) , 90-2, (1988)] |
|
N-bromoimide/DBU combination as a new strategy for intermole...
2013-10-18 [Org. Lett. 15(20) , 5186-9, (2013)] |
|
Colorimetric and titrimetric assay of isoniazid.
1992-06-01 [J. Pharm. Biomed. Anal. 10(6) , 421-6, (1992)] |
|
Site-selective bromination of vancomycin.
2012-04-11 [J. Am. Chem. Soc. 134(14) , 6120-3, (2012)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved