| Structure | Name/CAS No. | Articles |
|---|---|---|
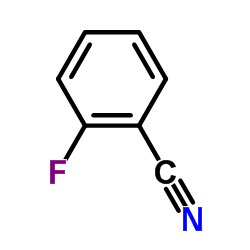 |
2-Fluorobenzonitrile
CAS:394-47-8 |
H M Colquhoun, D F Lewis, D J Williams
Index: Org. Lett. 3(15) , 2337-40, (2001)
Full Text: HTML
[reaction: see text] Condensation of 2-fluorobenzonitriles with phenoxides affords 2-aryloxybenzonitriles that cyclize cleanly in trifluoromethanesulfonic acid at room temperature to give xanthone-iminium triflates. The C=N bond in these compounds is remarkably resistant to hydrolysis, but prolonged reaction with strong aqueous acid under vigorous conditions affords xanthones in good yield. The synthesis is exemplified for a novel series of polynuclear dixanthones and for a high molar mass polyxanthone derived from the previously unreported monomer 3,3'-difluoro-4,4'-biphenyldicarbonitrile.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
2-Fluorobenzonitrile
CAS:394-47-8 |
C7H4FN |
|
High-sensitivity elemental ionization for quantitative detec...
2015-12-21 [Analyst 140 , 8177-85, (2015)] |
|
Studies on the synthetic compatibility of aryloxime linkers ...
2000-05-19 [J. Org. Chem. 65(10) , 2924-32, (2000)] |
|
Aminoborohydrides. 12. Novel tandem S(N)Ar amination-reducti...
2001-03-23 [J. Org. Chem. 66(6) , 1999-2004, (2001)] |
|
Efficient synthesis of 5-(4'-methyl [1, 1'-biphenyl]-2-yl)-1...
[J. Org. Chem. 58(18) , 5023-24, (1993)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved