| Structure | Name/CAS No. | Articles |
|---|---|---|
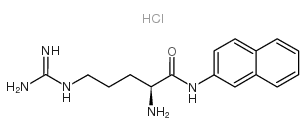 |
L-Arginine β-naphthylamide hydrochloride
CAS:18905-73-2 |
|
 |
NCI-83633
CAS:732-85-4 |