Microwave synthesis of cucurbit[n]urils.
Nial J Wheate, Nilesh Patel, Oliver B Sutcliffe
Index: Future Med. Chem. 2(2) , 231-6, (2010)
Full Text: HTML
Abstract
Cucurbit[n]urils (CB[n]; n = 5, 6, 7, 8 or 10) are a family of macrocycles made from the acid-catalyzed condensation of glycoluril and formaldehyde.The synthesis of CB[n] using microwave radiation has been examined and the effect of acid type, reaction time and temperature on the distribution of products has been determined. Synthesis in HCl yields CB[5], CB[6], CB[7] and CB[8] in 10 min and is most efficient at 160 °C. Synthesis in H(2)SO(4) yields mostly CB[6] in 3 min and is most efficient at 160 °C.Microwave synthesis provides an efficient and cost-effective method for the large-scale production of CB[n] for a range of applications, particularly drug delivery.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
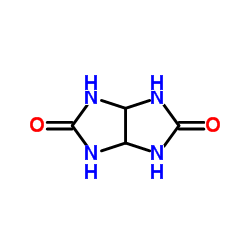 |
Glycoluril
CAS:496-46-8 |
C4H6N4O2 |
|
Molecular clips and tweezers hosting neutral guests.
2011-01-01 [Chem. Soc. Rev. 40(1) , 30-43, (2011)] |
|
Encapsulation of ion pairs in extended, self-assembled struc...
2012-07-25 [J. Am. Chem. Soc. 134(29) , 11971-3, (2012)] |
|
New supramolecular organization for a glycoluril: chiral hyd...
2002-10-07 [Chem. Commun. (Camb.) (19) , 2228-9, (2002)] |
|
Efficient DNA condensation induced by ruthenium(II) complexe...
2012-12-14 [Chemistry 18(51) , 16383-92, (2012)] |
|
Synthesis of glycoluril catalyzed by potassium hydroxide und...
2010-01-01 [Ultrason. Sonochem. 17(1) , 55-7, (2010)] |