Immunoaffinity isolation of the sulfate conjugate of 4'-hydroxypropranolol from plasma.
T D Eller, U K Walle, T Walle
Index: J. Chromatogr. A. 612(2) , 320-5, (1993)
Full Text: HTML
Abstract
Selective extraction of sulfate conjugates of basic drugs from biological matrices has been difficult because of their highly polar nature. Immunoaffinity isolation may be the best solution to this analytical problem. This was tested for a model compound, the metabolite 4'-hydroxypropranolol sulfate (HOPS), which was effectively extracted from plasma by a column containing antibodies to the parent drug propranolol. The specificity was very high, giving little interference from the biological material in subsequent high-performance liquid chromatographic analysis with fluorometric detection. The method for HOPS was highly reproducible and provided a sensitivity of 1 ng/ml plasma. The technique was applied to measurements of HOPS in plasma after therapeutic doses of propranolol as well as to the individual enantiomers after chiral derivatization.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
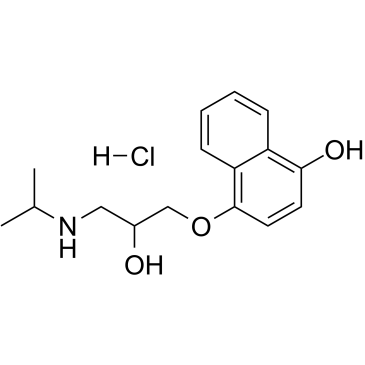 |
4-hydroxy propranolol hcl
CAS:14133-90-5 |
C16H22ClNO3 |
|
Stereoselective high-performance liquid chromatography deter...
1997-04-25 [J. Chromatogr. B. Biomed. Sci. Appl. 692(1) , 133-40, (1997)] |
|
Potential bias and mitigations when using stable isotope lab...
2010-01-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878(31) , 3267-76, (2010)] |
|
Potent antioxidant properties of 4-hydroxyl-propranolol.
2004-01-01 [J. Pharmacol. Exp. Ther. 308(1) , 85-90, (2004)] |
|
Hep G2 cell line as a human model for sulphate conjugation o...
1992-08-01 [Xenobiotica 22(8) , 973-82, (1992)] |
|
Involvement of SULT1A3 in elevated sulfation of 4-hydroxypro...
2005-03-15 [Biochem. Pharmacol. 69(6) , 941-50, (2005)] |