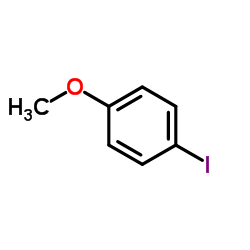
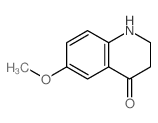
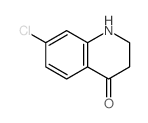
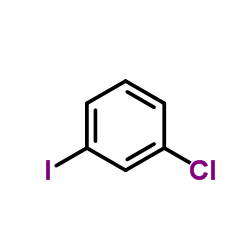
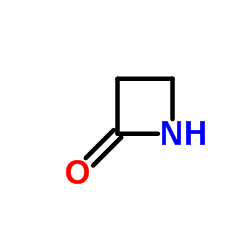
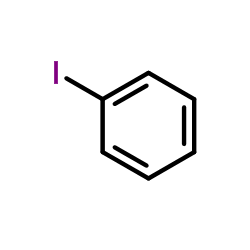
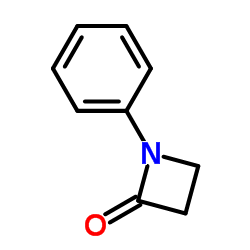
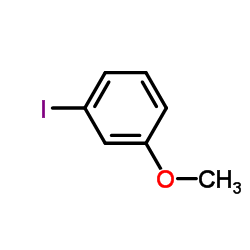
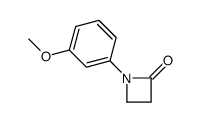
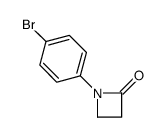
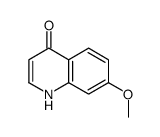
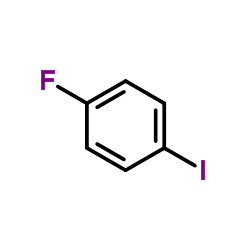
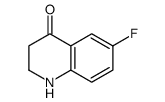
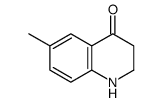
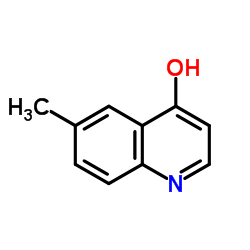
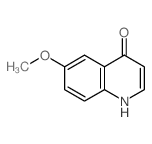
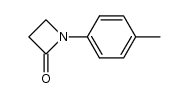
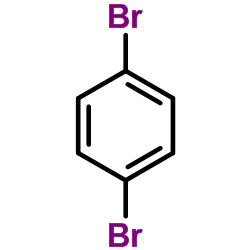
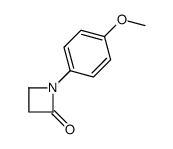
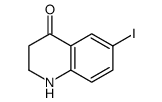
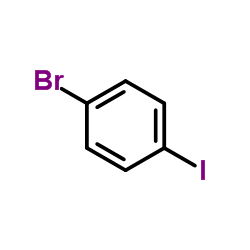
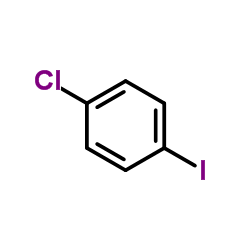
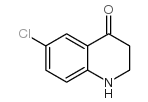
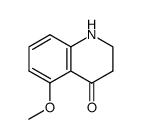
|
~% |
|
~50% |
|
~% |
|
~82% |
|
~98% |
|
~92% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~88% |
|
~95% |
|
~% |
|
~38% |
|
~94% |
|
~95% |
|
~% |
|
~% |
|
~66% |
|
~% |
|
~97% |
|
~% |
|
~% |