| Structure | Name/CAS No. | Articles |
|---|---|---|
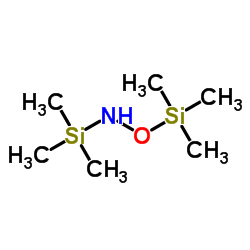 |
N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE
CAS:22737-37-7 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE
CAS:22737-37-7 |