Journal of Colloid and Interface Science
2002-11-15
Effects of aminoacids on the autooxidation of 3,5-di-tert-butylcatechol in the presence of surface-active copper(II) complexes.
Y Y Lim, L P Liew
Index: J. Colloid. Interface Sci. 255(2) , 425-7, (2002)
Full Text: HTML
Abstract
The rate of autooxidation of 3,5-di-tert-butylcatechol (3,5-DTBC) in the presence of micelles formed from mixing equal concentrations of [Cu(C(12)-tmed)Br(2)] (where C(12)-tmed is N,N,N'-trimethyl-N'-dodecylethylenediamine) and several amino acids has been investigated. It was found that the rate in air-saturated solution is very much dependent on pH, which affects the availability of copper(II) coordination site for the catechol and the degree of micellization. At a given pH, the rates in [Cu(C(12)-tmed)Br(2)] micellar media are greatly enhanced in the presence sodium halide.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
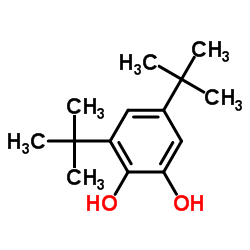 |
3,5-Di-t-butylcatechol
CAS:1020-31-1 |
C14H22O2 |
Related Articles:
More...
|
Catechol oxidase activity of a series of new dinuclear coppe...
2008-08-18 [Inorg. Chem. 47(16) , 7083-93, (2008)] |
|
3,5-Di-t-butyl catechol is a potent human ryanodine receptor...
2012-07-01 [Pharmacol. Res. 66(1) , 80-7, (2012)] |
|
A novel tripodal ligand containing three different N-heteroc...
2009-01-01 [Chemistry 15(22) , 5567-76, (2009)] |
|
Chemistry of singlet oxygen--48. Isolation and structure of ...
1987-09-01 [Photochem. Photobiol. 46(3) , 325-30, (1987)] |
|
3,5-di-t-butylcatechol (DTCAT) as an activator of rat skelet...
2005-02-01 [Biochem. Pharmacol. 69(3) , 485-91, (2005)] |