[A common and insidious side-effect: allergic contact dermatitis caused by bufexamac used in the treatment of dermatitis. Results from the Information Network of Departments of Dermatology (IDVK)].
A Schnuch, O Gefeller, W Uter
Index: Dtsch. Med. Wochenschr. 130(50) , 2881-6, (2005)
Full Text: HTML
Abstract
Bufexamac is a non-steroidal, anti-inflammatory drug used in the topical treatment of atopic dermatitis, stasis dermatitis and perianal eczema. The substance is known to cause severe allergic contact dermatitis (ACD) as an adverse effect (AE), which may be indistinguishable from the eczema which is to be treated. Hence the diagnosis of this AE is often considerably delayed. In order to estimate the quantitative importance of ACD to bufexamac, data of the Information Network of (German) Departments of Dermatology (IVDK) from July 1999 to December 2004 were analysed.During the study period, 39,392 unselected patients from 40 German departments of the IVDK were patch tested with bufexamac (5 % pet). The results of the reading after 72 hours were analysed. The dichotomized patch test result was further assessed for possible risk factors from the patients' history and clinical diagnosis by Poisson regression analysis.In 560 of 39,392 patients contact allergy to bufexamac was diagnosed, i. e. 1.4 % (95 % confidence interval: 1.3 - 1.5), standardized for sex and age. The Poisson regression analysis revealed a significantly increased risk associated with the following factors: multiple sensitization, perianal eczema, underlying atopic dermatitis, leg dermatitis, female gender and residence in areas of Germany other than Eastern Germany. The latter observation can be explained by low prescription rates in Eastern Germany.Bufexamac is an important allergen. Extrapolating the frequency of 1.4 % in our data to the whole German population by the CE-DUR approach yields an estimate of about 6000 cases per year. In view of the high frequency of sensitization, the pitfalls in diagnosis, the severity of the course of disease and the lack of efficacy of this drug, the risk to benefit ratio is obviously critical.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
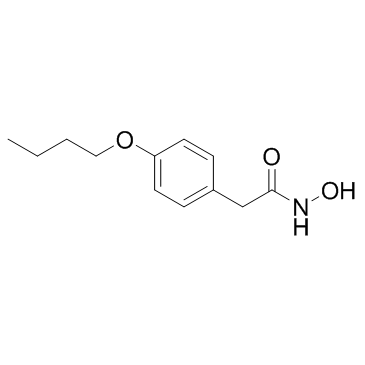 |
Bufexamac
CAS:2438-72-4 |
C12H17NO3 |
|
Erythema-multiforme-like, urticarial papular and plaque erup...
2015-02-11 [Contact Dermatitis 31(2) , 97-101, (1994)] |
|
[A severe epicutaneous test reaction to the bufexamac in a h...
1999-10-08 [Dtsch. Med. Wochenschr. 124(40) , 1168-70, (1999)] |
|
In vitro photobiochemical characterization of sulfobutylethe...
2011-06-01 [J. Pharm. Biomed. Anal. 55(3) , 591-6, (2011)] |
|
Examinations of the antioxidative properties of the topicall...
2003-10-01 [J. Pharm. Pharmacol. 55(10) , 1379-88, (2003)] |
|
Allergic contact dermatitis to topical preparations of bufex...
2012-08-01 [Australas. J. Dermatol. 53(3) , 207-10, (2012)] |