Experimental and theoretical studies of magnetic exchange in silole-bridged diradicals.
Nans Roques, Philippe Gerbier, Ulrich Schatzschneider, Jean-Pascal Sutter, Philippe Guionneau, José Vidal-Gancedo, Jaume Veciana, Eva Rentschler, Christian Guérin
Index: Chemistry 12(21) , 5547-62, (2006)
Full Text: HTML
Abstract
Five bis(tert-butylnitroxide) diradicals connected by a silole (7 a-d) or a thiophene (12) ring as a coupler were studied. Compound 12 crystallizes in the orthorhombic space group Pna2(1) with a = 20.752(5), b = 5.826(5), and c = 34.309(5) A. X-ray crystal structure determination, electronic spectroscopy, variable-temperature EPR spectroscopy, SQUID measurements and DFT computations (UB3LYP/6-31+G*) were used to study the molecular conformations and electronic spin coupling in this series of molecules. Whereas compounds 7 b, 7 c, and 7 d are quite stable both in solution and in the solid state, 7 a and 12 undergo a partial electronic rearrangement to both a diamagnetic quinonoidal form and a monoradical species owing to the fact that they correspond to the open form of a pi-conjugated Kekulé structure. In the solid state, magnetic measurements indicate that the diradicals are all antiferromagnetically coupled, as expected from their topology. These interactions are best reproduced by means of a "Bleaney-Bowers" model that gives values of J = -142.0 cm(-1) for 7 a, -1.8 cm(-1) for 7 b, -1.3 cm(-1) for 7 c, -4.2 cm(-1) for 7 d, and -248.0 cm(-1) for 12. The temperature dependence of the EPR half-field transition in frozen dichloromethane solutions is consistent with singlet ground states and thermally accessible triplet states for diradicals 7 b, 7 c, and 7 d with DeltaE(T-S) values of 3.48, 2.09, and 8 cm(-1), respectively. No evidence of a populated triplet state was found for diradicals 7 a and 12. Similarities between the DeltaE(T-S) and J values (DeltaE(T-S) = -2 J) clearly show the intramolecular origin of the observed antiferromagnetic interaction. Analyses of the data with a "Karplus-Conroy"-type equation enabled us to establish that the silole ring, as a whole, allows a more efficient magnetic coupling of the two nitroxide radicals attached to its 2,5-positions than the thiophene ring. This superiority probably originates from the nonaromaticity of the silole which thus permits a better magnetic interaction through it. DFT calculations also support the experimental results, indicating that the magnetic exchange pathway preferentially involves the carbon pi system of the silole.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
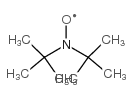 |
di-tert-butyl nitroxide
CAS:2406-25-9 |
C8H18NO |
|
Using cyclodextrins to encapsulate oxygen-centered and carbo...
2010-06-03 [J. Phys. Chem. A 114(21) , 6217-25, (2010)] |
|
Stratum corneum intercellular lipid as compared to erythrocy...
1995-01-01 [Physiol. Chem. Phys. Med. NMR 27(1) , 63-72, (1995)] |
|
Guest inclusion in cucurbiturils studied by ESR and DFT: the...
2012-08-23 [J. Phys. Chem. A 116(33) , 8475-83, (2012)] |
|
Model membrane partition ESR study in the presence of alpha-...
1989-08-01 [Biosci. Rep. 9(4) , 489-95, (1989)] |
|
Calorimetric and electron spin resonance examination of lipi...
1988-11-01 [J. Invest. Dermatol. 91(5) , 499-505, (1988)] |