Chemistry-based risk assessment for skin sensitization: quantitative mechanistic modeling for the S(N)Ar domain.
D W Roberts, A O Aptula, G Y Patlewicz
Index: Chem. Res. Toxicol. 24(7) , 1003-11, (2011)
Full Text: HTML
Abstract
There is a strong impetus to develop nonanimal based methods to predict skin sensitization potency. An approach based on physical organic chemistry, whereby chemicals are classified into reaction mechanistic domains and quantitative models or read-across methods are derived for each domain, has been the basis of several recent publications. This article is concerned with the S(N)Ar reaction mechanistic domain. Electrophiles able to react by the S(N)Ar mechanism have long been recognized as skin sensitizers and have been used extensively in research studies on the biology of skin sensitization. Although qualitative discriminant analysis approaches have been developed for estimating the sensitization potential for S(N)Ar electrophiles on a yes/no qualitative basis, no quantitative mechanistic model (QMM) has so far been developed for this domain. Here, we derive a QMM that correlates skin sensitization potency, quantified by murine local lymph node assay (LLNA) EC3 data on a range of S(N)Ar electrophiles. It is based on the Hammett σ(-) values for the activating groups and the Taft σ* value for the leaving group. The model takes the form pEC3=2.48 Σσ(-) + 0.60 σ* - 4.51. This QMM, generated from mouse LLNA data, provides a reactivity parameter 2.48 Σσ(-) + 0.60 σ*, which was applied to a set of 20 compounds for which guinea pig test results were available in the literature and was found to successfully discriminate the sensitizers from the nonsensitizers. The reactivity parameter correctly predicted a known human sensitizer 2,4-dichloropyrimidine. New LLNA data on two further S(N)Ar electrophiles are consistent with the QMM.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
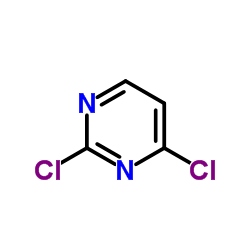 |
2,4-Dichloropyrimidine
CAS:3934-20-1 |
C4H2Cl2N2 |
|
Novel vanilloid receptor-1 antagonists: 1. Conformationally ...
2007-07-26 [J. Med. Chem. 50 , 3497, (2007)] |
|
One-pot Double Suzuki Couplings of Dichloropyrimidines.
2012-02-01 [Synthesis 2010(16) , 2721-2724, (2010)] |
|
An efficient route to 4-aryl-5-pyrimidinylimidazoles via seq...
2006-01-19 [Org. Lett. 8(2) , 269-72, (2006)] |
|
Selective iron-catalyzed cross-coupling reactions of grignar...
2004-05-28 [J. Org. Chem. 69 , 3943-3949, (2004)] |
|
Synthesis and SAR of aminopyrimidines as novel c-Jun N-termi...
2007-06-15 [Bioorg. Med. Chem. Lett. 17 , 3463, (2007)] |